Details of the Drug
General Information of Drug (ID: DMFWMKZ)
| Drug Name |
ZK-93423
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ZK 93423; 83910-44-5; ZK93423; ZK 93 423; 6-Benzyloxy-4-methoxymethyl-beta-carboline-3-carboxylic acid ethyl ester; 9H-Pyrido(3,4-b)indole-3-carboxylic acid, 4-(methoxymethyl)-6-(phenylmethoxy)-, ethyl ester; ALBKMJDFBZVHAK-UHFFFAOYSA-N; PDSP1_001754; AC1L3TNV; AC1Q64YI; CHEMBL10947; GTPL4346; SCHEMBL7117282; CHEMBL1518572; BDBM85039; CHEBI:92593; ZINC5857871; PDSP2_001737; PDSP1_001771; ethyl 6-(benzyloxy)-4-(methoxymethyl)-9h-; BDBM50007553; PDSP2_001754; CAS_121926; NSC_121926
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
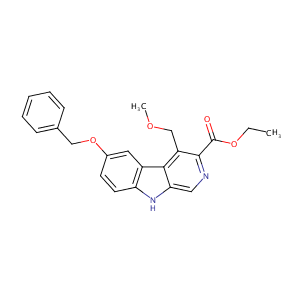 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 390.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
References
